�o���O�s�[���A�O�o�j�Ǯɪ��ͤƽҤW������LNADH�b�q�l�ǻ��� (Electron transfer chain)�W�PATP���ഫ�v�A�o�Ӱ��D�]�b�Ұ�W�Q�Q�L�C�������ͤƽҥ��j�h�H1��NADH�ഫ3��ATP�A��1��FADH2�h�ഫ2��ATP�A�G���p�U�Ѹ���}�}�l���㪺�}�ѧ@�ΩҲ��ͪ�ATP�|��38�ӡA���̪ͤƽҥ����H1��NADH�ഫ2.5��ATP�A��1��FADH2�ഫ1.5��ATP�A�G�P�˪��������i�o�쪺ATP�ƶq�h�u��32�ӡA�G��ӵ��׳���I�H�U���������ഫ������M��]�G
�b���M�[������������C�Ĥ� (oxidative phosphorylation) ���ƾǰ��X�Ҧ� ( chemiosmotic model) ���U�A�Ұ��]����������{���p�U�G
xADP + xPi + 1/2 O2 + H* + NADH ---> xATP + H2O + NAD*
(��: �]�������X�W�СA�G��*�������l)
�ӥH�W���Y��"x"�A���ɺ٬� P/O ratio �� P/2e- ratio---�û�����ơC
�H�W�� Lehninger Principles of Biochemistry, 4ed. p.712�Ҵy�z���[���A�Ӧb�t�@��textbook (Biochemistry, Campbell& Farrell. 4ed. p.560) ���h����P/O ratio ���q�l�ǻ���ATP�ͦ������X��A�Ҧp�b 1/2 O2 + 2H* +2e- ---> H2O ���������A�C�P�� 1 more ���l�b
ADP + Pi ---> ATP ���������ҷ|���Ӫ� Pi ���ռ�. (Pi�����O phosphate ion)�C²��ӻ��A�N�O 1��NADH�g�L����C�Ĥƪ��L�{��A�i��o 3�� ATP �A���� NADH �� P/O ratio��3�C
�\�h������w�p��X��NADH���q�l�����̮ɡA��P/O ratio ����2-3�A�Ӱ��]P/O ratio ������ơA�j����������̦P�N NADH �� P/O ratio��3�A�Ӫ�X�~�o�Ǽƭȫh�X�{�b���m�P�Ь�Ѥ��C�bLehninger�o���Ѥ����йq�l�ǻ����ƾǺ��z���XATP�X�����d�ҡA�õL�z�׳W�w P/O ratio �n����ơC�Ӭ������ƾǭp�q�����D�h�ܦ��F"�q�@�� NADH ���q�l�ǻ����𪺹L�{���A���X�ӽ�l (�Y�B���l)�|�Q pump �ܽ��~�H�ӤS���X�ӽ�l�����z�LF0F1 complex �y�i�����H�X�ʤ@��ATP���Φ��H�n���q��l���y�ʦb�N�W�ܽ��� (�o�q�������y�z�b�����حz)�ANADH �C�@��q�l�npump out �X���~����l�ƶq��10�ӡA��FADH�h��6�ӡC�ӭn�X�ʤ@��ATP���ͩһݪ���l�ơA�̼s������������ƾڬ�4�ӡA�Ө䤤�@�ӥΨӿ�ePi, ATP�MADP �q�L�ɽu�齤�C���]�C�� NADH�b�q�l�ǻ��줤��10�ӽ�l�Qpump out�A�Ӧ��|�ӥ���flow in �Ӳ���ATP�A�h NADH �� proton-based P/O ratio �h��2.5 (10���H4)�A��FADH�� P/O ratio �h��1.5 (6���H4)�C�bLehninger���ͤƽҥ����ϥΪ�P/O ratio ��2.5�M1.5�A���ϥξ��2.0�M3.0���ͤƤ��m���ܴ��M�C
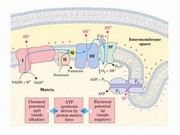
�����q�l�ǻ��쪺�ܷN�ϡA�`�N�bO2�o��q�l���e�A�q����pump�X�h��H+�@��10�ӡA�ӤW�z���ƾǺ��z���X�A�N�O�k�䨺�ӹ��q�O�w��complex�A���ⳡ���J�ս�A���O�OFo�MF1�A�����O�Q�ν����~����l�@�t(��l��סFproton gradient)���X��ATP�����͡C |